THE FRAGILE X SYNDROME
This condition is the commonest inherited cause of mental retardation.
Incidence
The Fragile X syndrome affects approximately 1 in 2000 males and accounts for 4-8% of all males with mental retardation.
Clinical features
Older boys and adult males usually have a recognisable facial appearance with high forehead, large ears, long face and prominent jaw. After puberty most affected males have large testes (macro-orchidism). There can also be evidence of connective tissue weakness with hyperextensible joints, stretch marks on the skin (striae) and mitral valve prolapse. The mental retardation is moderate to severe and many affected boys show autistic features and/or hyperactive behaviour. Speech tends to be halting and repetitive. Female carriers can show some of the facial features and approximately 50% of women with the full mutation show mild to moderate retardation.
The Fragile X chromosome
The Fragile X syndrome takes its name from the appearance of the X chromosome which shows a fragile site close to the telomere at the end of the long arm at Xq27.3. A fragile site is a non-staining gap usually involving both chromatids at a point at which the chromosome is liable to break. In this condition, detection of the fragile site involves the use of special culture techniques such as folate or thymidine depletion, which can result in the fragile site being detectable in up to 50% of cells from affected males. Demonstration of the fragile site in female carriers is much more difficult and cytogenetic studies alone are not a reliable means of carrier detection, in that although a positive result confirms carrier status, the absence of the fragile site does not exclude a woman from being a carrier.
The molecular defect
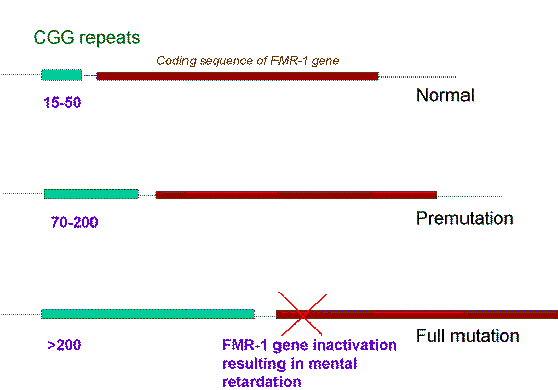
The Fragile X locus is known as FRAXA. The FRAXA mutation consists of an increase in the size of a region in the 5’-untranslated region of the Fragile X mental retardation (FMR-1) gene. This region contains a long CGG trinucleotide repeat sequence. In the DNA of a normal person there are between 10 and 50 copies of this triplet repeat and these are inherited in a stable fashion. However, a small increase to between 50 and 200 renders this repeat sequence unstable, a condition in which it is referred to as a premutation.

A man who carries a premutation is known as a ‘normal transmitting male’. All of his daughters will inherit the premutation and will be of normal intelligence, but when these daughters come to have sons there is a high risk that the premutation will undergo a further increase in size during meiosis. If this reaches a critical size of greater than 200 CGG triplets it becomes a full mutation. This process is sometimes referred to as expansion of the triplet repeat sequence.
The full mutation is unstable not only during female meiosis but also in somatic mitotic divisions. Consequently in an affected male, gel electrophoresis shows a ‘smear’ of DNA consisting of many different sized alleles rather than a single band. Note that a premutation can be identified by PCR, whereas Southern blotting is necessary to detect full mutations as the long GCC expansion is refractory to PCR amplification.
At the molecular level a full mutation suppresses transcription of the FMR-1 gene by hypermethylation, and this in turn is thought to be responsible for the clinical features seen in males, and in some females with a large expansion. The FMR-1 gene contains 17 exons which encode a cytoplasmic protein which plays a crucial role in the development and function of cerebral neurones.
The Fragile X syndrome – genotype-phenotype correlations
|
Number of triplet repeats (Normal range = 10-50) |
Fragile site detectable |
Intelligence |
|
Males 50-200 (premutation) |
No |
Normal (normal transmitting male) |
|
200-2000 (full mutation) |
Yes (in up to 50% of cells) |
Moderate to severe mental retardation |
|
Females 50-200 (premutation) 200-2000 (full mutation) |
No Yes (usually <10% of cells) |
Normal 50% normal, 50% mild mental retardation |
Genetic counselling and the Fragile X syndrome
This common cause of mental retardation presents a major counselling problem. The normal rules of X-linked Inheritance can appear to be modified.
All the daughters of a normal transmitting male will carry the premutation. Their male offspring are at risk of inheriting either the premutation or a full mutation. This risk is dependent on the size of the premutation in the mother, with mutations greater than 100 CGG repeats almost invariably increasing in size to become full mutations.
For a woman who carries a full mutation there is a risk of 1 in 2 that each of her sons will be affected with the full syndrome and that each of her daughters will inherit the full mutation. Since approximately 50% of females with the full mutation are mildly retarded, the risk that a female carrier of a full mutation will have a retarded daughter equals ½ x ½, i.e. ¼. Prenatal diagnosis can be offered based on analysis of DNA from chorionic villi.